Advanced Therapies
More
We use cookies to offer you the best experience on our site. You can find out more about the cookies we use or disable them in the Cookie settings
Begin with the end in mind: adopt the right fill & finish strategy to support all stages of your product development and to timely deliver your therapies to the patients
AT-Closed Vial®
Discover more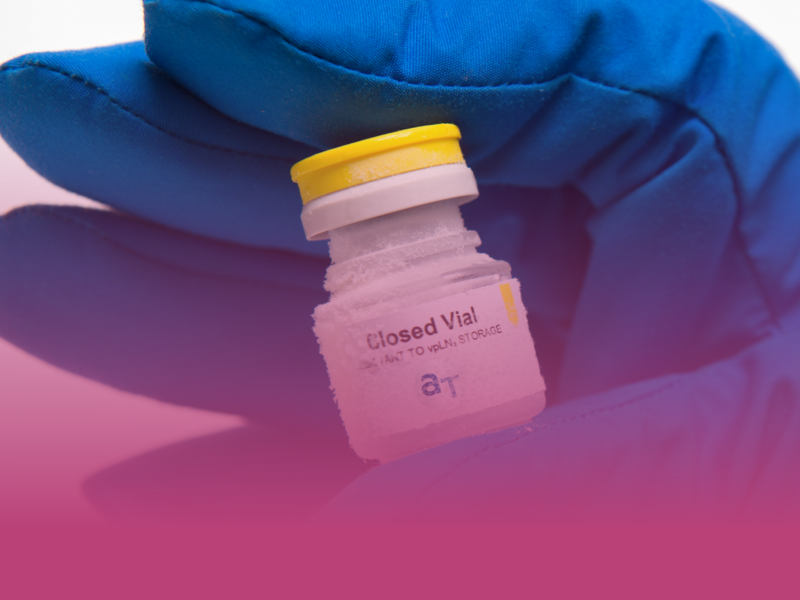
Aseptic filling machines
Discover more
LifeCycle Support
Discover more
Ancillary equipement
Discover more
More than just a filling machine: it is a patented and fully validated filling process, based on a polymer vial with unique features
The vial is closed before being filled: when produced, the AT-Closed Vial® is molded and stoppered in ISO 5 environment, and when processed in the fill & finish suite, it is filled through the stopper
Both less and more: it is naturally scalable and fits personalized and off-the-shelf therapies
Zero-defect philosophy: de-risked process with minimized product loss
The “future is now” mindset: holistic approach to making the cutting-edge treatments, as cell and gene therapy, available to the patients, from development to commercial phase. Widely used for the advanced therapies (ATMP) since 2009.
