AT-Closed Vial® and Container Closure Integrity during Cryogenic Storage
More
We use cookies to offer you the best experience on our site. You can find out more about the cookies we use or disable them in the Cookie settings
Since 2002 at Aseptic Technologies we explore ways to make aseptic fill & finish process safer and easier, responding to new challenges of biopharmaceutical injectable developers and their CDMOs.
We fundamentally re-invented the entire process of fill & finish starting with its core element: the vial or more precisely the entire container closure system.
The AT-Closed Vial® and the AT-Closed Vial® Technology Filling Lines are a result of that reflection.
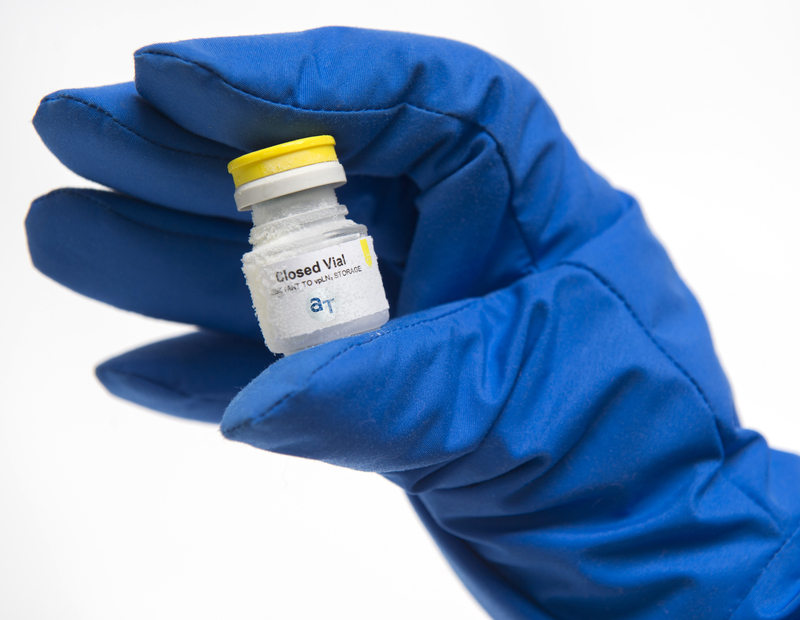
The AT-Closed Vial® is a vial that is closed before filling. The vial body and stopper are assembled together right after molding, in ISO 5 conditions. The vials are therefore closed before the start of the fill & finish process, eliminating contamination risks. Its unique material composition and manufacturing technique also enables the AT-Closed Vial® to secure Container Closure Integrity (CCI) during cryopreservation.

The closed vial filling is performed in 3 steps with dedicated filling equipment in ISO 5 :

The AT-Closed Vial® technology enables safe storage of the injectable drug product and its intermediate GMP-grade products at low temperatures.
The robustness of the container closure integrity (CCI) in cryogenic temperatures is validated and holistically covered by the vial materials and design, its manufacturing process, and the closed vial filling process.
Combined with the rapidity of operation for minimizing of the contact with the cryoprotectant, the AT-Closed Vial® technology is, since 2009, the solution of choice for safe storage at low temperatures of autologous and allogeneic cell and gene therapies, vectors, cell banks and trusted by pharmaceutical companies, academic institutions, CDMOs and hospitals globally.

Together with our users on a journey of making the novel injectable treatments a commodity, at Aseptic Technologies, we provide the following features for the cost of goods reduction, while operating with AT-Closed Vial® technology:
In addition, the AT-Closed Vial® is available in three formats in order to satisfy your specific needs: AT-CryoBox (9 to 25 vials depending on the vial volume), AT-Nest (25 to 88 vials) and AT-Tray (91 to 377 vials).
AT-Closed Vial® and Container Closure Integrity during Cryogenic Storage
More