
We use cookies to offer you the best experience on our site. You can find out more about the cookies we use or disable them in the Cookie settings
The AT-Cellyx is an integrated equipment solution designed to optimize the final processing of cell therapies. It uniquely combines controlled mixing and cooling in a single, compact device, ensuring optimal product homogeneity and thermal stability. This innovation significantly reduces manual handling and seamlessly integrates into existing fill-finish setups, making it a simple, ready-to-use system for consistent, reproducible results from the first to the last drop.

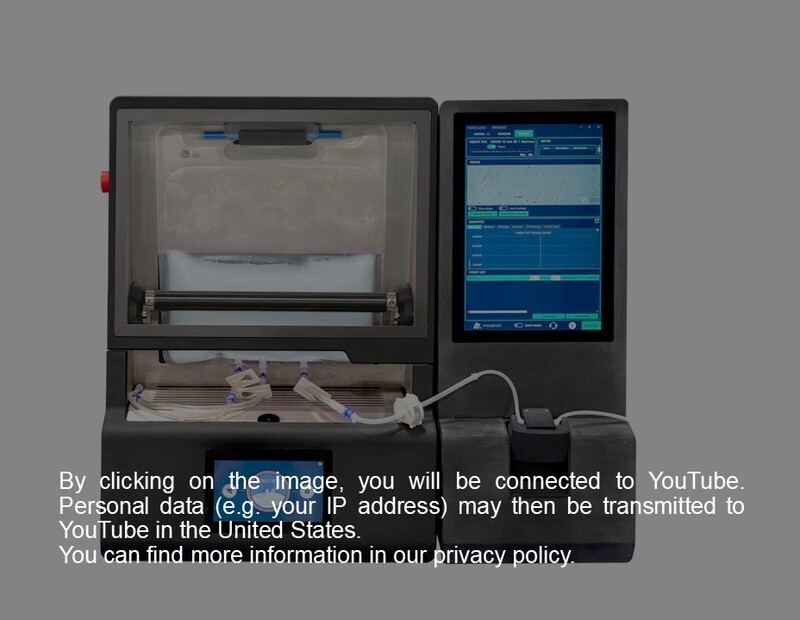
The AT-Cellyx streamlines the final processing of cell therapy products through a controlled, integrated sequence.