AT-Closed Vial® and Container Closure Integrity during Cryogenic Storage
More
We use cookies to offer you the best experience on our site. You can find out more about the cookies we use or disable them in the Cookie settings
Begin with the end in mind: adopt the right fill & finish strategy to support all stages of your product development and to timely deliver your therapies to the patients
Container Closure Integrity at low temperatures
Learn about CCI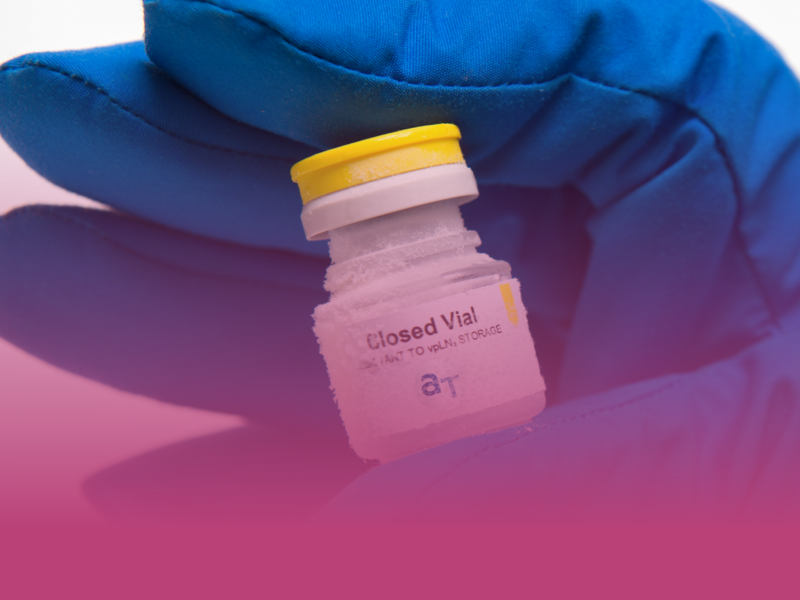
Cell & Gene Therapy
Discover more
Next Gen Biotech
Discover more
Compounding
Discover more
Clean injection molding
Discover more
AT-Closed Vial® and Container Closure Integrity during Cryogenic Storage
More